Abstract
Introduction: Chemo-immunotherapy with R-CHOP is the standard of care for newly diagnosed DLBCL of all ages leading to high cure rates although treatment-related toxicities are significant among older adults. Prior studies have shown that pre-treatment skeletal muscle mass and/or density can identify older adults at risk of adverse treatment outcomes. We sought to construct a risk-prediction model for severe treatment-related toxicities among older adults with DLBCL receiving chemotherapy by incorporating clinico-demographic features and skeletal muscle mass measurements.
Methods: We conducted a single institutional retrospective review of all adults ≥60y with newly diagnosed DLBCL receiving R-CHOP-based regimens between 1/2015 and 2/2021. We extracted baseline clinico-demographic variables (age, sex, race, albumin, ECOG performance status [PS], Charlson comorbidity index [CCI], stage, Revised International Prognostic Index score, growth factor use) and treatment intensity (dose at 1st cycle). Dose reduction was defined as any pre-planned reduction in or elimination of doxorubicin from standard CHOP-based regimens during cycle 1 of treatment. The primary outcome of interest was grade ≥3 treatment-related toxicity (using CTCAE v5.0) or mortality occurring within 30 days after each cycle. Treatment response was defined by Lugano criteria. Skeletal muscle area (SMA) and skeletal muscle density (SMD) were measured with mid-L3 computed tomography images using Data Analysis Facilitation Suite (DAFS) software (Voronoi Analytics, Canada) and was manually verified. Using forward stepwise binary logistic regression models (entry p <0.10, removal p <0.20), we developed a risk prediction model for grade ≥3 toxicity; putative risk factors included age, sex, ECOG PS, CCI, albumin, treatment intensity, myosteatosis, and sarcopenia. The performance of our prediction model was assessed using area under the curve receiver operating characteristics (AUC-ROC) (for discrimination) and Hosmer-Lemeshow goodness of fit probability plots (for calibration). Results were internally validated in 10-fold cross validation as well as 1000 random bootstrapped samples, and an optimism corrected AUC was estimated.
Results: Of 193 adults ≥60y with new DLBCL initiating chemo-immunotherapy during the study period, 168 completed their first cycle of treatment at our institution and were included in our study cohort. The median age was 72y (IQR 67-77) with 57% males and 83% White patients, 42% with stage IV disease, and 9% with double- or triple-hit subtype. Of those with reported ECOG PS, 38% had ECOG PS ≥2 and 29% had CCI ≥2.
Overall, 54% of patients received planned reduced dose treatment, most commonly in the >80y population in which 86% received ≥50% doxorubicin reduction. Over half (57%) experienced grade ≥3 toxicity (50% hematologic toxicity, 36% non-hematologic toxicity). Dose delays (16%), reductions (18%), or discontinuations (15%) were frequent, and over a third (39%) of patients had unplanned hospitalizations during their treatment course. Treatment-related mortality was <10%. At end of treatment, 63% achieved complete response, 18% achieved partial response, 3% had stable disease, and 12% had progressive disease.
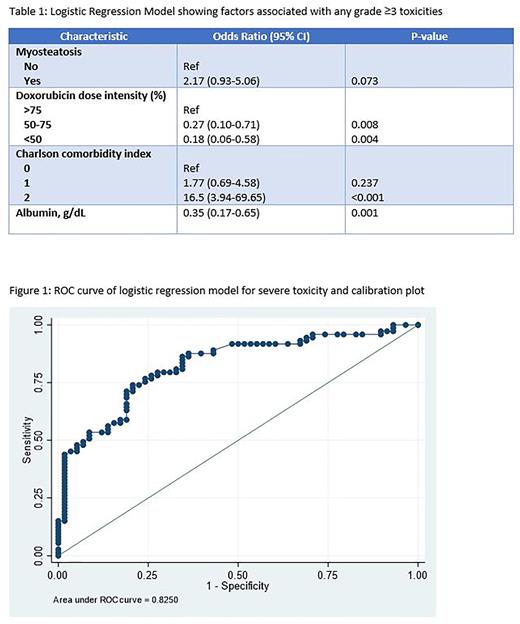
In logistic regression models, myosteatosis but not sarcopenia was associated with an increased risk of grade ≥3 non-hematologic toxicities (OR 2.30; 95% CI 1.03-5.16; p value 0.04) and a trend towards increased risk of grade ≥3 overall toxicities (OR 2.17; 95% CI 0.93-5.06; p value 0.07) (Table 1). A risk model incorporating myosteatosis, CCI, treatment intensity, and serum albumin had an AUC of 0.82 in predicting severe toxicity during therapy (Figure 1). Calibration plots and Hosmer-Lemeshow test (p value 0.33) suggested excellent model fit. Internal validation yielded similar results with an excellent discrimination (optimism corrected AUC of 0.78).
Conclusions: Among older adults ≥60y with DLBCL, severe treatment-related toxicities were common. A risk prediction model utilizing albumin, comorbidity burden, myosteatosis, and treatment intensity was able to reliably predict older adults at increased risk of severe toxicity. If independently validated in external datasets, our results can inform treatment selection for older adults with DLBCL undergoing first-line chemo-immunotherapy. Future work will examine prediction of early mortality and survival.
Disclosures
Goyal:Sutro Biopharma: Research Funding; SeaGen: Research Funding; UpToDate: Patents & Royalties; Viracta Therapeutics: Research Funding; 2nd.MD: Consultancy. Narkhede:TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; EUSA pharmaceuticals;: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Gilead/Forty-seven: Research Funding; Genmab: Research Funding; Genetech: Research Funding; Roche: Research Funding; Seagen Inc.: Research Funding. Mehta:I-MAB: Research Funding; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Roche-Genentech: Research Funding; Norvartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Research Funding; Juno pharmaceuticals/BMS: Consultancy, Research Funding; Kite/Gilead: Consultancy, Research Funding; Affimed: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; fortyseven Inc./Gilead: Consultancy, Research Funding, Speakers Bureau; BeiGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Innate pharmaceuticals: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Morphosys/Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Giri:OncLive: Honoraria; Pack Health: Research Funding; CareVive: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal